Roche and Alzheimer’s disease

SPECIAL REPORT 25.09
Roche and Alzheimer’s disease
On July 28, at the Alzheimer’s Association International Conference (AAIC) held in Toronto (Canada), Roche presented new data from its development portfolio including additional diagnostics outcomes.
In several oral presentations Roche disclosed its latest results from the ongoing trontinemab’s Phase Ib/II Brainshuttle AD study, as well as plans of the Phase III clinical study design.
Latest data from the trontinemab study, at the doses of 1.8 mg/kg and 3.6 mg/kg, showed rapid and robust reduction of amyloid plaques as measured by amyloid PET scans. In the 3.6 mg/kg cohort trontinemab reduced amyloid levels below the 24 centiloid positivity threshold in 91% of participants (n=49/54). These results were reinforced by significant reductions in fluid biomarkers including total tau, phosphorylated (pTau)181, pTau217 and neurogranin in cerebrospinal fluid (CSF) and plasma. Amyloid-related imaging abnormalities/effusions (ARIA-E) were only observed in less than 5% of participants (n=4/149).
The Swiss company has announced its plans for the Phase III studies, TRONTIER 1 and 2, which will start at the end of the year, investigating safety and efficacy of trontinemab in people with early Alzheimer’s disease. Primary endpoint will measure the change in cognition and function based on Clinical Dementia Rating-Sum of Boxes (CDR-SoB) scale after 18 months of treatment. Secondary endpoint will include assessment of cognition, function, behavioural symptoms and quality of life.
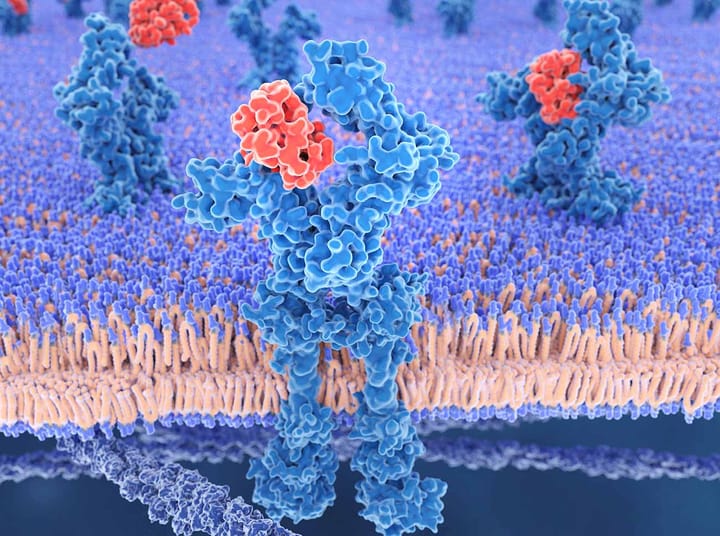
Trontinemab is an investigational brainshuttle bispecific amyloid-beta monoclonal antibody specifically engineered for enhanced access to the brain to enable rapid reduction of amyloid in people with Alzheimer's disease. Trontinemab is designed for the efficient transport across the blood-brain barrier to target aggregated forms of amyloid beta and remove amyloid plaques in the brain. The uniqueness of the technology results in high central nervous system exposure of trontinemab leading to a rapid and deep amyloid clearance.
New and real-world data showed that Elecsys pTau217 diagnostic test is equivalent to PET scans and CSF tests but can be performed with a simple blood sample and analysed in a routine clinical laboratory. The Elecsys pTau217 test is becoming a standalone blood test for rule-in and rule-out identification of amyloid pathologies with the potential to become standard-of-care clinical practice and thus remove current invasive interventions as well as stressing cognitive and imaging tests for Alzheimer’s disease patients.
This document has been prepared by Jean-Claude Muller and is provided for information purposes only. The information contained herein has been obtained from sources believed to be reliable but is not warranted to be accurate or complete. The views presented are those of the author at the time of writing and are subject to change. Jean-Claude Muller has no obligation to update these opinions or the information presented.




Comments ()